
About us
About Porton Biopharma
A long history of pharmaceutical development.
We specialise in the development and manufacture of biopharmaceuticals for niche indications.

Our History
Located in the heart of Wiltshire, Porton Biopharma has a rich history in the development and manufacture of biopharmaceuticals for a wide variety of diseases.
- 1951 — MoD Microbiological Research Establishment opened
- 1979 — Ownership passed to the UK government Department of Health & Anthrax Vaccine licensed in the UK
- 1985 — Erwinase first licensed in the UK
- 2013 — The site became the centre of Public Health England
- 2015 — PBL spun out of PHE to run as a commercial entity, whilst still owned by the Department of Health
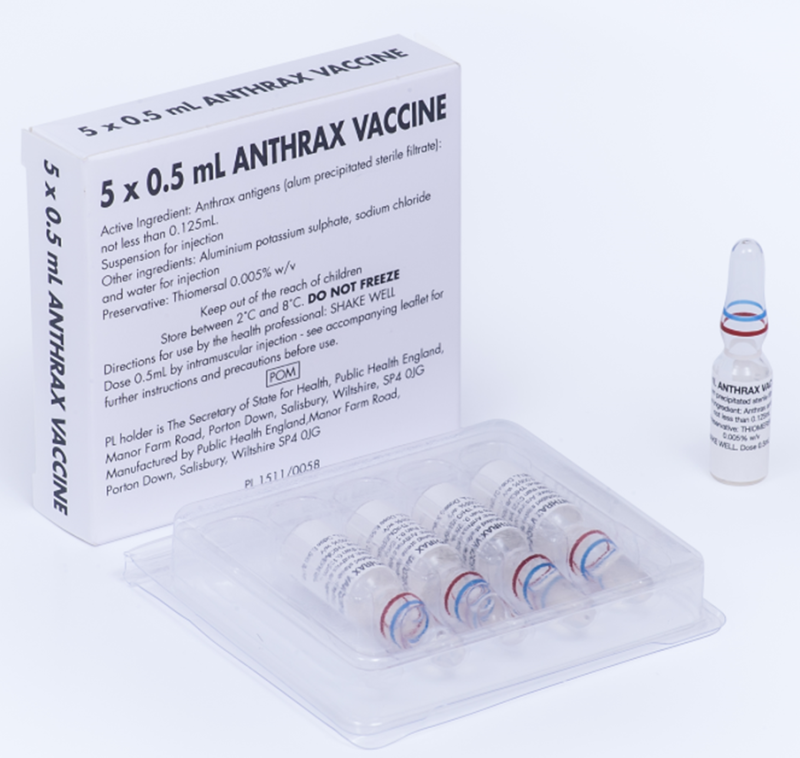
Anthrax Vaccine
We manufacture the UK’s licensed anthrax vaccine. It is supplied for both occupational health purposes and to protect those at risk from the use of anthrax as a biological weapon.

Erwinase (Crisantaspase)
Developed at Porton in the 1960’s, PBL remains the sole global manufacturer of Erwinase® which was first registered as a product name in the UK in 1985. Since then it has been supplied to treat haematological malignancies around the world.
Our Facilities
Expertise in microbial fermentation is central to what we do.
- A new state-of-the-art fermentation facility which has been designed and built around a 3000 litre fermenter, giving a significant increase in capacity.
- Dedicated containment facilities ensure both product and operator safety.
These new facilities, together with our commitments to develop and maintain our highly trained staff, ensure that we are able to meet the latest regulatory standards and continue treating our patients

Our Team

Mike Austin
Chief Executive Officer
Mike thrives in an environment of business challenge and ethical transparency, matched with the reward of making a difference in the provision of innovative medicines to patients.
Find out more
Matthew Way
Director of Supply Chain
Matt has over 30 years’ experience in Supply Chain & Logistics, gained across several industries. The majority of Matt’s career has been in the Military with extensive experience in supporting global ...
Find out more
Laura Ayling
Director of Commercial Partnerships and Strategy
Laura has over 15 years’ experience across the pharmaceutical industry, from Research & Development through to commercial supply
Find out more
Gaelle Davies
Lead Qualified Person
Gaelle is a member of the Royal Society of Chemistry, nominated as a Qualified Person under the provisions of Directive 2001/83/EC for Erwinase and Anthrax vaccine for over 15 years.
Find out more
Ian King
Head of Engineering
Ian has over 20 years of engineering experience and has performed both operational and project delivery roles in a range of organisations across the pharmaceutical sector.
Find out more
Angela Taylor
Head of Human Resources
Angela brings over 20 years experience in HR from a wide variety of sectors.
Find out moreBecome part of the team
If you are a motivated person who is interested in working in a high-tech environment as part of a dynamic team, then there could be a role for you.
- © 2024 Porton Biopharma
- Website by blue bee
- Terms of Use
- Privacy Policy
- Cookie Policy
Registered in England and Wales, company number 9331560. Registered office at Manor Farm Road, Porton Down, Salisbury, Wiltshire SP4 0JG. VAT number is GB 206 6518 18.